Busy day at MOLDX with three new draft LCDs and one new final LCD.
NEW DRAFT LCDS
DRAFT - ADVANCED PROSTATE
See DL39636, Gene expression profile (GEP) tests for decision making in castration resistant and metastatic prostate cancers.
MolDx has a long history of LCDs for prostate cancer prognostic tests, this appears to be a new version (not style as a revision). Prior versions focused on early diagnosis and management. This LCD is based on a February 2022 letter from Dr Davincioni of Decipher/Veracyte. MolDx publishes only the cover letter of the request.
The LCD notes it does not deal with CGP (e.g Foundation Medicine type tests) or "single biomarker" tests. Patients must be facing multiple therapy optoins of variable severity and the test will help make that choice.
The LCD does not named covered or non covered tests but the LCD has an associated billing article DA59462. This lists only 81479 as a potentially covered code, so that's kind of a dead end for policy wonks trying to determine or understand coverage (e.g. by doing studies on payer coverage and publishing them.)
On a commerical note, the Decipher Veracyte prostate GEP test has been taking a larger share of a market that once was divided in thirds (Veracyte, Genomic Health, Myriad). A Veracyte Decipher collaboration with Duke was just covered in Genomeweb based on a publication in JCO Precision Oncology by Bitting et al.
DRAFT LUNG NODULES
DL39654, Molecular biomarkers for risk stratification of indeterminate pulmonary nodules following bronchoscopy.
Note that this isn't framed as a policy for imaging-based nodules, but for nodules "following bronchoscopy." While this sounds like, for example, a Veracyte Percepta test, the LCD is framed as "internally initiated." The rules confirm this is for post bronchoscopy patients. The LCD separately discusses "first generation" and "second generation" Percepta tests.
The billing article DA59476 again presents itself as "information that complements the LCD" but simply lists 81479 as a possible billing code.
For the use case you might have thought of - a mystery small nodule on low dose CT screening or an incidental small lung nodule - and it's before bronchoscopy or PET - Medicare has an LCD for "XL2" test from Biodesix, here.
DRAFT THYROID NODULES
LCD DL39646 is "Risk Stratification for Thyroid Nodules" and is based on a letter from Veracyte (Dr Gerrard) from January 2022. The letter notes that coverage was previously requested for "Veracyte MTC" and that the January 2022 update restyles the request in the format of a foundational (pan-product) LCD. Coverage is valid for a patient with an indeterminate nodule Bethesda III or IV, or else, a nodule Bethesda V "for which molecular testing may aid in further decision making."
The LCD concludes, Given the cost and risks associated with thyroid surgery, the clinical decision making that goes into the extent of surgery, additional follow up management, and published guidelines, this contractor finds that molecular tests that aid in medical decision making for thyroid nodules of Bethesda Categories III-V are reasonable and necessary. Specifically, this contractor recognizes improved outcomes by safely precluding unnecessary surgical procedures with these services as well as better selecting patients who may benefit from such procedures.
Reference to specific tests in this document does not imply coverage by this contractor.
Readers unfamiliar with "foundational LCDs" may be puzzled by the remark that evaluation of a specific test in an LCD "does not imply coverage."
A billing article is provided, DA59470. Potentially covered codes include 81479, again, as well as specific code 81546. Veracyte GSC Genomic Sequencing Classifier.
NEW FINAL LCD: FLIP TO COVERAGE
FINAL RHEUMATOID ARTHRITIS
LCD L39424. This is based on a letter from SCIPHER, making PRISM-RA, The request letter is undated and the PDF has a document creation of 7/2022, so it might have been the date preceding the October 2022 draft LCD. While it's an inferenec, SCIPHER's approach for an LCD likely preceded the CAC in December 2021.
The original Draft LCD position on non coverage, in October 2022, was curt. It followed a CAC meeting in December 2021.
- "Current molecular biomarker tests to guide targeted therapy selection in Rheumatoid Arthritis (RA) are non-covered by this contractor."
Champagne corks are popping somewhere because the final LCD provides "limited coverage" based on "new evidence" and following 12 rules. The patient has a history of failure or contraindication to at least one first line DMARD and is considering a range of alternate therapies (TNF, JAK, etc). And MolDx has determined the test to be appropriately validated and designed, etc.
The billing article - again - simply points to a possible unlisted code, in this case, 81599, other MAAA test. See A59529.
With the flip in coverage status, there is a lengthy "response to comments" document A59519. I extract and highlight the specific rationale for the favorable policy change, and critiques of the field that MolDx still feels are valid. Sidebar here. (There, I also ask ChatGPT for its opinion on the policy flip.)
MY COMMENT
IImproved Coordination & Timing for Comment
This crop of LCDs, draft and final, had better coordination of posting, with the same timing at the several MolDx MACs - which will help speed up finalization of those in draft. Draft LCD have comment from August 31 to October 14.
For two dated LCD request letters, the time from letter to a draft LCD was 6-9 months. The time from draft to final LCD, for the one final LCD, was 10 months.
Convergent Evolution of LCD Text
The LCDs for indeterminate nodules (lung, thyroid, etc) tend to consolidate toward very similar language in terms of coverage and performance rules. Eg. rules on the lines of, "the test must be appropriate designed for the population."
Stymies Academics and Publications
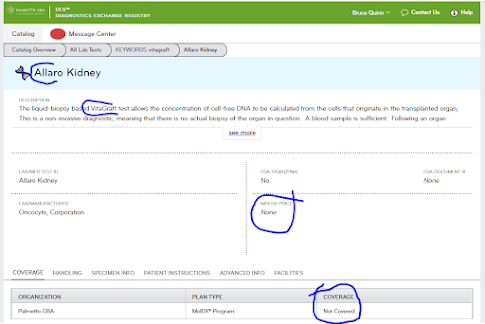
There's been excellent work in recent years documenting and publishing the nature of payor policies, but policy experts with be stymied by the lack of specific coverage details either in LCDs or articles. To some extent, the DEX database run by Palmetto can back fill this, but includes no coverage dates and no archival form (DEX lives in the eternal present moment only). This limits the abilities of academic experts, or consultancies, to tabulate coverage issues, even if they are on grants commissioned by HHS. See eg Trosman et al. 2020 PMID 32389219.
PAMA Thwarted?
The widespread use of "unlisted codes" puts whole fields of genomics outside the elaborately designed CMS and Congressional PAMA pricing system, even as genomics grows as a proportion of lab spending.
I ask GPT 3.5 about the R.A. policy flip [lower part of the linked page]. Here.