On July 6, 2023, the FDA released its favorable approval decision for LEQEMBI, lecanemab, from Eisai/Biogen.
CMS only covers amyloid drugs under a registry, and CMS unveiled web pages for a rapid easy patient registry as well as one clinical trial design using that registry.
To the extent they have stated coverage, MACs have generally non-covered APOE testing for Alzheimer's disease. The FDA labeling has a black box warning requiring APOE4 testing, so MACs will likely accommodate that quickly. Details below.
The FDA Labeling
Find the 22 page FDA label here:
https://www.accessdata.fda.gov/drugsatfda_docs/label/2023/761269s001lbl.pdf
- As with most FDA labels, the indication is simple. "Leqembi is indicated for the treatment of Alzheimer's disease. Patients should, at initiation, have mild cognitive impairment MCI or mild dementia."
Since FDA released Aduhelm earlier with no comment on disease stage, the FDA indication for Leqembi immediately notes that this mild population was the population studied in the clinical trials.
A black box warning discusses ARIA (brain edema), notes it is more likely in ApoE4 homozygotes, says gene testing SHOULD be done, and closes by saying that risks should be considered before prescribing Leqembi.
See the FDA press release here.
Amyloid Testing?
Does the labeling require amyloid PET scans or other testing, like CSF testing? Yes, though it's not in the top line indication, when you get to "Dosage and Administration" it quickly says, presence of beta-amyloid pathology must be confirmed.
But how? This sentence now guides us to Section 2.1 which is empty except to forward the reader to 12.1. But 12.1 is simply the "Mechanism of action" section which says that this is an antibody directed against amyloid-beta. So as a guide to the physician, or payer, so far, what beta amyloid tests FDA expects, it's pretty hopeless. If you flip forward on your own to 14, Clinical Studies, it simply says there that studies were conducted on "patients with confirmed presence of amyloid pathology."
So if you were a doctor wondering which amyloid tests to order, or a payor researching which ones to cover, you'd hit a dead end in several different ways.
Based on simple searches, the term CSF occurs only once or twice, and the word "cerebrospinal" does not occur.
___
But, the CMS CED study description here requires confirmation by "PET, CSF, or Blood tests." There are FDA approved PET and CSF biomarkers, but no FDA approved blood tests.
ApoE4 Testing
CPT only has a generic Tier 2 code, 81401, for genotyping ApoE (meaning type 2,3,4). It pays $137.
It's generally not covered or not paid at NGS MACs and MOLDX MACs.
On the other hand, well, take a deep breath. A lot was paid at the Novitas/First Coast MACs, in 2021, but they had no working edits on Tier 2 codes, a fact that was vigorously criticized by the OIG recently (here) (my comment here).
One Novitas/Texas lab had $7M in billings for 81401, which was half of US billings. The rest is generally in other obscure labs down in the Novitas MAC.
The lab with the highest 81401 billing also had sky high Tier 2 billings in general, including a massive $100M for 81408 (near half the US total for 81408). In short, we can't conclude much from Tier 2 Code 81401 usage at CMS.
I think that MACs will need to orient to paying for 81401 in bona fide Alzheimer cases in line for Leqembi, and this genetic test should merit its own Tier 1 CPT code. There's no such code in the 2023 code book nor in the Summer 2023 CMS pricing sessions, suggesting that no ApoE code was created as late as May 2023.
CMS CED STUDY
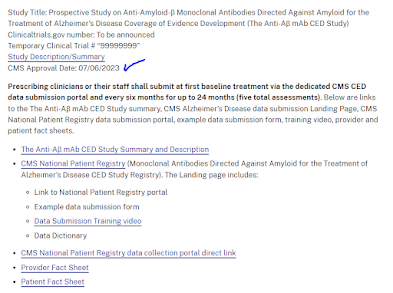
CMS promised to post a registration site and a first, foot in the door, registry for Leqembi on FDA Day One. CMS met its goal.
Here's the page for CED studies on amyloid drugs, highlighting the new study opened today.
There's a "study description," a "Data submission training video," etc. Cognition will be reported every 6 months using "MoCA or Another Instrument." Info on interim diagnosis of ARIA will also be reported. CMS expects to extracts measures of "slow in decline of cognition" although it's hard to define "slowing" with no past history and no control group. Similarly, CMS will defined "incidence of brain edema and hemorrhage" although recall that in the FDA data, these have a quite significant incident in the control (no drug) group as well, and the CMS study has no control group.
Study requires PET, CSF, or "blood test" confirmation for entry; see earlier remarks on testing. The cognitive test is listed as "MoCA or other." Function should be assessed by the FAQ function test. The study is purportedly "sized" by "precentage of subjects with function [FAQ] >3 points" by two years, but this is not in comparison to anything, so it's odd as an "outcome" measure - outcome compared to what??
There are some quality metrics for physician where they get points for participating in registries; this may include that. Training video here.
CED Falls Flat?
CMS recently published an elaborate guidance to CED document, with high expectations and standards. This CED study seems to meet none of them.
It's an colossal example of form over content (here), literally. The protocol says, yes, the primary outcome is to "assess whether MAbs meaningfully IMPROVE health outcomes," and the measure for this is FAQ - Functional Activities Questionaire - for "statistically significant stability in function." But the measure has nothing to compare against, so it's nonsensical as a "proof of improved clinical outcome." There's no clue what the predefined statistics are for the linear measure in a single observational population are. There's a nod to "statistics" in a crytpic "statistical sample size" table and a promise that endpoints will be "statistically meaningful" without the actual work of showing that is possible with the tools available or the data availalble.
##
While they seem to put more weight on the FAQ functional assessment test, they also ask for a one-page, 15 minute test Montreal Cognitive Assessment, which is sort of a society-page version of the Mini Mental Status Test MMSE. Here. It tests things like remembering 5 objects, knowing the date and city, drawing a clock. The FAQ also takes 15 minutes, uses an informant, and quizzes things like "shopping alone, preparing a balanced meal, writing checks."
I suspect both tests could be suspected of being culturally biased. The MoCA is designed as a cutoff-based screening tool, not a longitudinal tracking tool (here; O'Driscoll 2017).