On January 12, 2023, CMS opened an NCD on coverage of injectable PrEP drugs for HIV prevention.
The action has to do with nuances of Medicare law. Medicare can cover preventive services endorsed by USPSTF, and PrEP has been endorsed by USPSTF at least since 2019. However, Medicare preventive services NCDs are only undertaken for Part A (hospital) and Part B (physician) services. The original PrEP drugs were oral drugs, and were not physician services.
In December 2021, FDA approved APRETUDE, an injectable HIV prevention drug given every two months. In February 2022, ViiV submitted a detailed coverage request letter. It seems that CMS had to wait a year, until USPTF released at least a draft version of a positive rating for new PrEP therapies, including APRETUDE. The drug is a 600 mg slow-release suspension of capotegravir in an IM/gluteal injection.
I would expect that CMS will produce and finalize a favorable NCD. I would expect they will write it in an open ended way to automatically cover FDA-approved and physician-injected HIV prevention drugs endorsed by USPSTF in the future.
The NCD tracking sheet is here:
CMS introductory text states:
- Per Section 1861(ddd) of the Social Security Act and implementing regulations at 42 CFR 410.64, CMS may cover "additional preventive services," if it determines through the national coverage determination (NCD) process that the service is recommended with a grade A (strongly recommends) or grade B (recommends) rating by the United States Preventive Services Task Force (USPSTF) and that it also meets certain other requirements.
- The USPSTF recently published (December 13, 2022) a draft recommendation with a grade A for prescribing PrEP with effective antiretroviral therapy to persons who are at increased risk of HIV acquisition to decrease the risk of acquiring HIV infection (https://www.uspreventiveservicestaskforce.org/uspstf/draft-recommendation/prevention-human-immunodeficiency-virus-hiv-infection-prep ). This draft is an update to their 2019 recommendation.
- CMS received a complete, formal request to consider an NCD for coverage of PrEP using antiretrovirals for persons at high risk of HIV acquisition.
- CMS is soliciting public comment relevant to the consideration. We are particularly interested in comments that include scientific evidence and that address the breadth of the request.
Comment is open to February 11, 2023, with a draft memo no later than July 12.
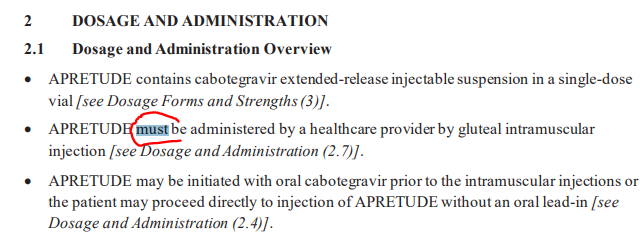
The bimonthly gluteal intramuscular injection modality (FDA) should protect the drug from being ruled a "self administered" drug by local MACs. CMS normally leaves S.A.D. decisions to local MACs, but I don't think there is any reason CMS cannot take an interpretation of the S.A.D. regulations and include it as part of the NCD benefit category. This may seem redundant but would cut off the chance of a contrary view by some nutty local medical director in the future. The words "must be administered by a health care professional" are indeed found on page 2 of the FDA instructions, which I was relieved to find. (See pic below).