From August 2020 to December 2021, the Trump then Biden administrations put out successive comment rounds and rulemaking announcements about "MCIT," Medicare Coverage for Innovative Technology," which provided 4 years of coverage at Medicare after a device was cleared through the breakthrough pathway (whether specifically de novo, 510(k), or PMA). The rule was finally canceled in November, see back story and links here.
However, the concept lives on as a section of the omnibus legislative package 21st Century Cures "Version 2022."
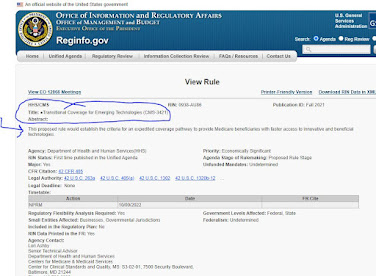
Meanwhile, the Office of Regulatory Affairs in the White House has posted a notice of future (eventual) rulemaking on the topic. The new policy, when it is finally crafted and proposed, will be retitled, "Transitional Coverage for Emerging Technologies." We read, "This proposed rule would establish the criteria for an expedited coverage pathway to provide Medicare beneficiaries with faster access to innovative and beneficial technologies." To be continued.
- See the placeholder webpage here.
This page will also list (via the "12866" link) any meetings held between public stakeholders (e.g. AdvaMed, etc) and the OMB.
 |
| click to enlarge |